
On May 31, 2025, Evopoint Biosciences — a platform-based innovative biopharmaceutical company committed to improving human health through cutting-edge pharmaceutical solutions— announced the first public release of key clinical data for its investigational Class 1 novel drug, Igermetostat (XNW5004), a selective EZH2 inhibitor, at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting. This milestone marks a significant step forward for Evopoint in strengthening its differentiated global position in hematologic oncology.
Abstract Title:
A Phase 1/2 Study to Evaluate the Safety and Efficacy of Igermetostat (XNW5004), a Selective EZH2 Inhibitor, in Subjects with Relapsed/Refractory Non-Hodgkin Lymphoma
Abstract Number: 7012
Presentation Type: Oral Presentation
Presentation Time: May 31, 2025, 8:00–9:30 AM (GMT-5)
The 2025 ASCO Annual Meeting was held in Chicago, USA. The ASCO Annual Meeting is the world’s premier academic event in oncology, spotlighting the latest advances in cancer research and clinical treatment each year.
Igermetostat Demonstrates Superior Efficacy in R/R FL Compared to Conditionally Approved Therapies
In the ongoing Phase I/II clinical trial of Igermetostat for relapsed/refractory non-Hodgkin lymphoma (R/R NHL), as of December 18, 2024, a total of 120 patients who had received at least two prior lines of systemic therapy were enrolled. The treatment was well tolerated, with no dose-limiting toxicities (DLTs) observed. Based on these findings, 1200 mg was selected as the recommended Phase II dose (RP2D).
Key outcomes at the 1200 mg dose include:
- In the relapsed/refractory follicular lymphoma (R/R FL) cohort (N=30), the overall response rate (ORR) was 66.7% ( Figure 1), with a disease control rate (DCR) of 100% and a median progression-free survival(mPFS) of 10.8 months.
- In patients with EZH2-mutant R/R FL, the ORR reached 70%.
In patients with EZH2 wild-type R/R FL, the ORR was 63.2%.
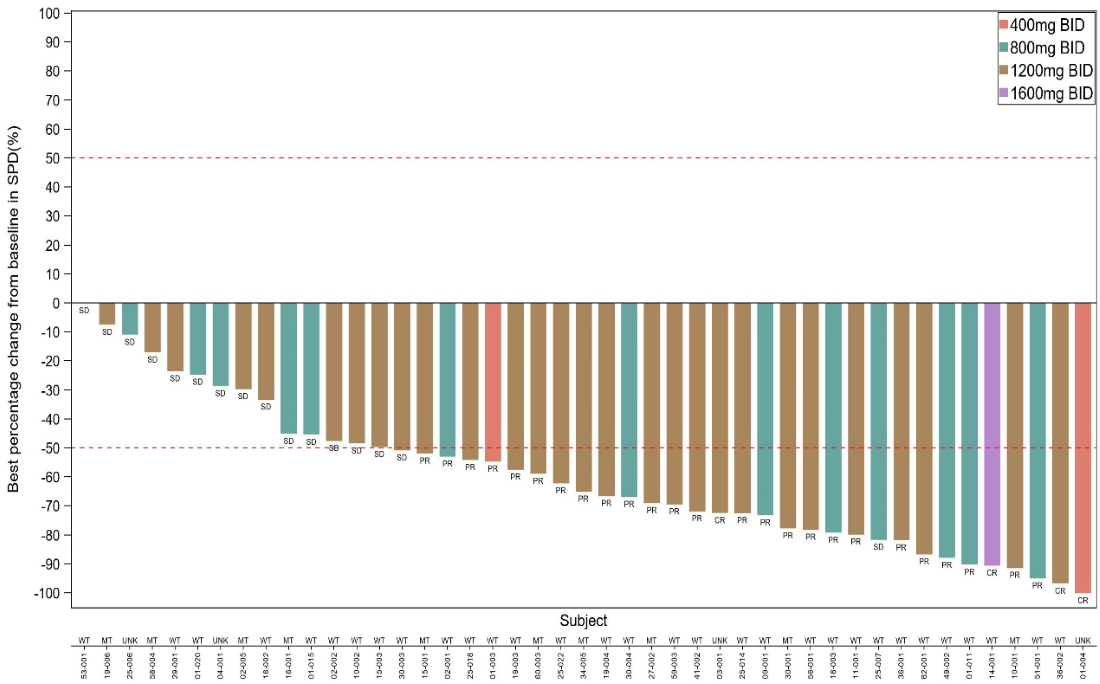
These results suggest that Igermetostat may offer superior clinical benefit compared to conditionally approved therapeutic agents in the same category. In May 2025, China’s Center for Drug Evaluation (CDE) granted Breakthrough Therapy Designation to Igermetostat for the treatment of R/R FL (EZH2 wild-type) in patients who have previously treated with at least three lines of systemic therapies.
Igermetostat Shows Promising Potential in R/R PTCL with Robust Clinical Outcomes
Compared to Approved or Investigational Drugs
Igermetostat also demonstrated compelling efficacy in patients with relapsed/refractory peripheral T-cell lymphoma (R/R PTCL) at the 1200 mg dose:
- The ORR in the R/R PTCL cohort (N=37) was 70.3% (Figure 2), with an mPFS of 15.7 months.
- In PTCL not otherwise specified (PTCL-NOS), the ORR was 72%.
In angioimmunoblastic T-cell lymphoma (AITL), the ORR was 68.2%.
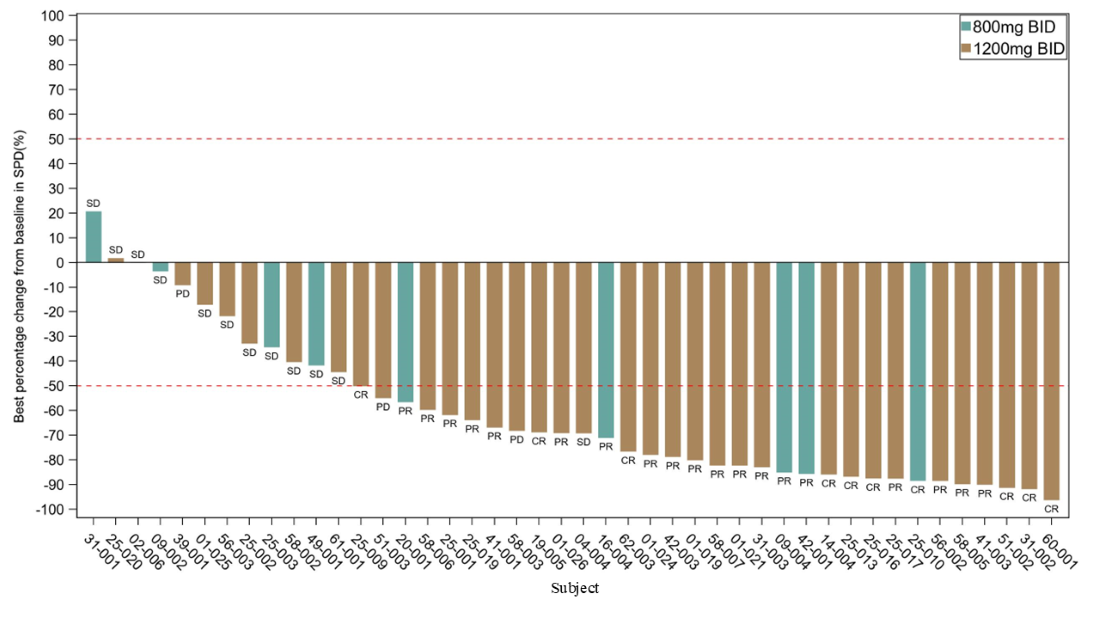
Igermetostat outperformed both approved and investigational therapies in this setting, showing superior tumor response rates and progression-free survival. In September 2024, the CDE granted Breakthrough Therapy Designation for Igermetostat in the treatment of R/R PTCL. A pivotal registration trial of Igermetostat for the treatment of peripheral T-cell lymphoma is currently underway.
Expert Perspective
Prof. Qiu Lugui
Chief Physician, Doctoral Supervisor
Chief Clinical Expert, Hematology Hospital, Chinese Academy of Medical Sciences
“The incidence of follicular lymphoma (FL) in China continues to rise. Igermetostat has shown remarkable anti-tumor activity in R/R FL patients, especially those previously treated with three or more systemic therapies, and provides clinical benefit regardless of EZH2 mutation status. It holds great promise as a genotype-independent EZH2 inhibitor for FL. PTCL accounts for over 20% of NHL cases in China. Igermetostat’s efficacy in R/R PTCL, particularly in the poor-prognosis PTCL-NOS subtype, significantly exceeds current treatments and could offer patients comprehensive benefits and improved treatment options. Additionally, combining Igermetostat with standard first-line regimens may reshape the future of PTCL therapy.”
About Igermetostat (XNW5004)
Igermetostat is a novel, selective, substrate-competitive small molecule inhibitor of EZH2, independently developed by Evopoint. Both preclinical and clinical studies have demonstrated its strong anti-tumor activity as monotherapy in relapsed/refractory follicular lymphoma and peripheral T-cell lymphoma. In combination with enzalutamide, Igermetostat has shown therapeutic synergy in metastatic castration-resistant prostate cancer without added safety risks. Currently, two registrational trials in lymphoma and one Phase I/II study in prostate cancer are in progress.
About EZH2
EZH2 is the catalytic subunit of the Polycomb Repressive Complex 2 (PRC2), which represses target gene transcription through methylation of histone H3 at lysine 27 (H3K27me3). It plays a critical role in regulating cell cycle progression, senescence, differentiation, and tumorigenesis. EZH2 mutations occur in 7–12% of FL cases and result in its constitutive activation, disrupting germinal center B-cell (GCB) and follicular helper T-cell (Tfh) interactions, impairing the germinal center response, and driving B-cell transformation. In EZH2 wild-type FL, somatic mutations in CREBBP and KMT2D may silence genes involved in B-cell egress from the germinal center, thereby maintaining EZH2 activity. EZH2 is also widely overexpressed in PTCL, and its expression is associated with increased tumor proliferation and poor prognosis. In prostate cancer, EZH2 functions as a transcriptional activator of the androgen receptor (AR) and its downstream pathways, and the increased EZH2 mRNA and protein expression is associated with accelerated disease progression.
Forward-Looking Statement
This press release contains forward-looking statements based on the company's current expectations and beliefs. Actual results may differ materially due to various risks and uncertainties.
*Note: This press release is for informational purposes only and does not constitute any investment advice. Evopoint does not endorse the use of any unapproved drug or indication. For more information about diseases, drugs, diagnosis, or treatment, please consult a qualified healthcare professional.





